Identification of TGFb - mediated M0 - homeostatic microglia
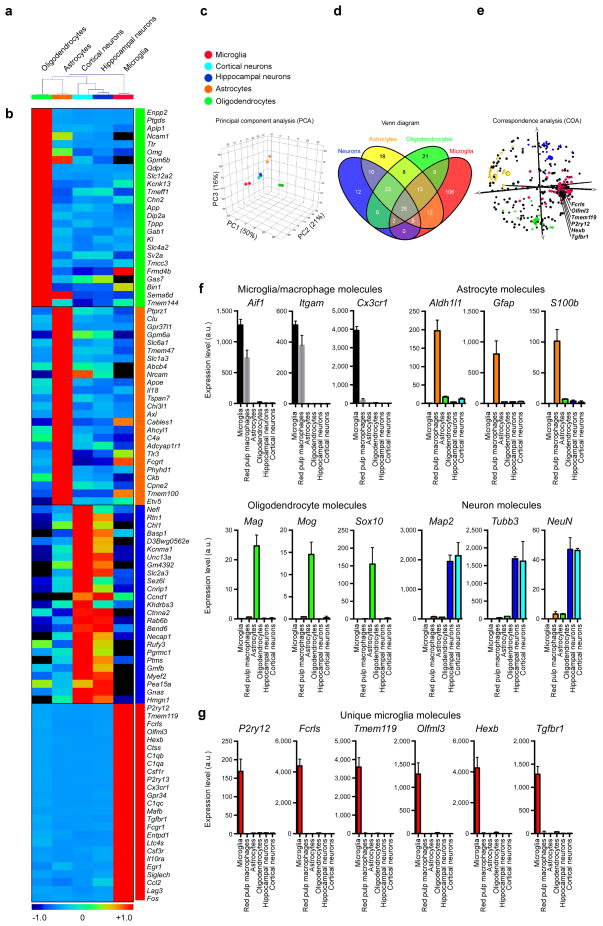
In 2014 our laboratory investigated the molecular signature of microglia and identified 239 genes and 8 microRNAs that were uniquely or highly expressed in microglia vs. myeloid and other immune cells. Out of 239 genes, 106 were enriched in microglia as compared to astrocytes, oligodendrocytes and neurons. This microglia signature was not observed in microglial lines or in monocytes recruited to the CNS and was also observed in human microglia. Based on this signature, we found a crucial role for TGF-β in microglial biology that included: 1) the requirement of TGF-β for the in vitro development of microglia that express the microglial molecular signature characteristic of adult microglia; and 2) the absence of microglia in CNS TGF-β1 deficient mice. Our results identify a unique microglial signature that is dependent on TGF-β signaling which provides insights into microglial biology and the possibility of targeting microglia for the treatment of CNS disease.
Identification of TREM2 - APOE mediated MGnD - neurodegenerative microglia

ABSTRACT
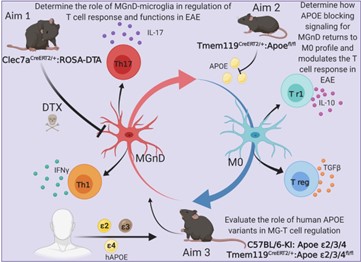
In 2017 Our laboratory finalized a project, led by, Susanne Krasemann and Charlotte Madore, in which We identified a specific apolipoprotein E (APOE)-dependent molecular signature in microglia from models of amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS) and Alzheimer’s disease (AD) and in microglia surrounding neuritic β-amyloid (Aβ) -plaques in human AD brains. The APOE pathway mediated a switch from a homeostatic to neurodegenerative microglia phenotype following phagocytosis of apoptotic neurons. Triggering receptor expressed on myeloid cells 2 (TREM2) induced APOE signaling, and targeting the TREM2-APOE pathway restored the homeostatic signature of microglia in ALS and AD mouse models and prevented neuronal loss in an acute model of neurodegeneration. APOE-mediated neurodegenerative microglia led to a loss in their tolerogenic function. Taken together, our work identifies the TREM2-APOE pathway as a major regulator of microglial functional phenotype in neurodegenerative diseases and serves as a novel target to restore homeostatic microglia.
→ READ MORE
Microglia in Glaucoma

Abstract
In 2022 Our laboratory finalized a project, led by Dr. Milicia Margeta, investigating the role of The mechanisim of apolipoprotein E4 (APOE4) in two mouse glaucoma models, microglia transitioned to a neurodegenerative phenotype characterized by upregulation of Apoe and Lgals3 (Galectin-3), which were also upregulated in human glaucomatous retinas. Mice with targeted deletion of Apoe in microglia or carrying the human APOE4 allele were protected from retinal ganglion cell (RGC) loss, despite elevated intraocular pressure (IOP). Similarly to Apoe-/- retinal microglia, APOE4-expressing microglia did not upregulate neurodegeneration-associated genes, including Lgals3, following IOP elevation. Genetic and pharmacologic targeting of Galectin-3 ameliorated RGC degeneration, and Galectin-3 expression was attenuated in human APOE4 glaucoma samples. These results demonstrate that impaired activation of APOE4 microglia is protective in glaucoma and that the APOE-Galectin-3 signaling can betargeted to treat this blinding disease.
Identification of IFNg - mediated pre - MGnD microglia
Abstract
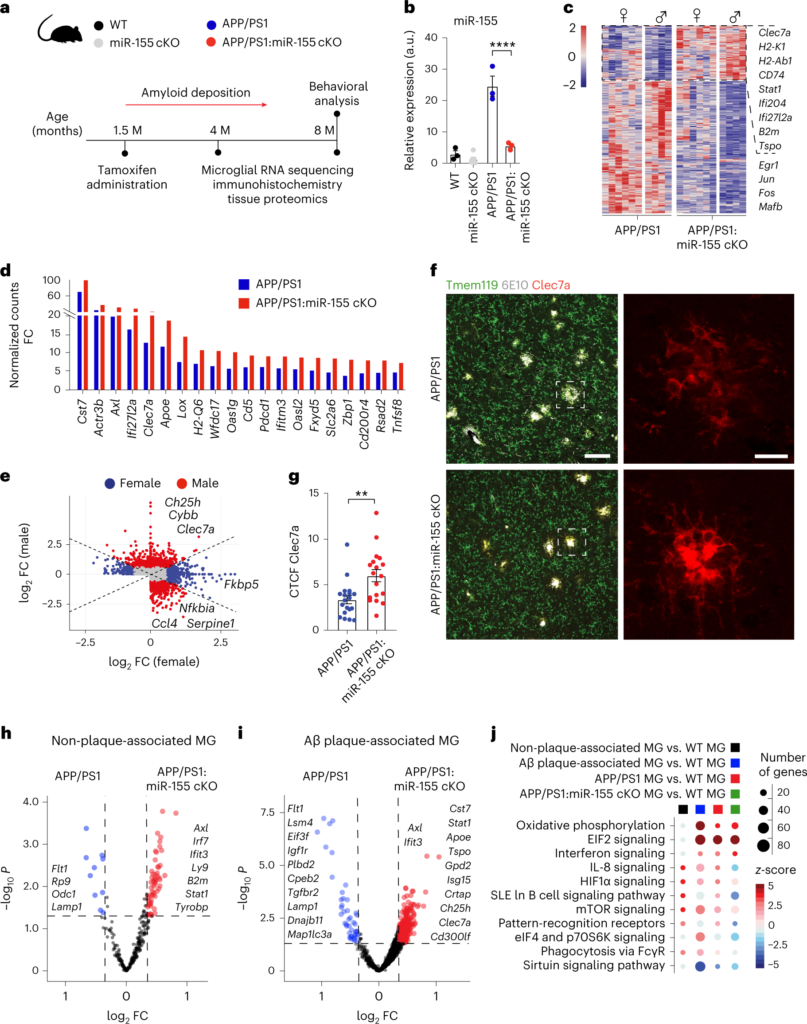
Microglia play a critical role in brain homeostasis and disease progression. In neurodegenerative conditions, microglia acquire the neurodegenerative phenotype (MGnD), whose function is poorly understood. MicroRNA-155 (miR-155), enriched in immune cells, critically regulates MGnD. However, its role in Alzheimer’s disease (AD) pathogenesis remains unclear. Here, we report that microglial deletion of miR-155 induces a pre-MGnD activation state via interferon-γ (IFN-γ) signaling, and blocking IFN-γ signaling attenuates MGnD induction and microglial phagocytosis. Single-cell RNA-sequencing analysis of microglia from an AD mouse model identifies Stat1 and Clec2d as pre-MGnD markers. This phenotypic transition enhances amyloid plaque compaction, reduces dystrophic neurites, attenuates plaque-associated synaptic degradation and improves cognition. Our study demonstrates a miR-155-mediated regulatory mechanism of MGnD and the beneficial role of IFN-γ-responsive pre-MGnD in restricting neurodegenerative pathology and preserving cognitive function in an AD mouse model, highlighting miR-155 and IFN-γ as potential therapeutic targets for AD.
Microglia in Multiple Multiple sclerosis (MS)
ABSTRACT
Generation of microglia - specific mAbs (4D4, P2ry12, FCRLS)

Microglia are myeloid cells of the central nervous system (CNS) that participate both in normal CNS function and disease. We investigated the molecular signature of microglia and identified 239 genes and 8 microRNAs that were uniquely or highly expressed in microglia vs. myeloid and other immune cells. Out of 239 genes, 106 were enriched in microglia as compared to astrocytes, oligodendrocytes and neurons. This microglia signature was not observed in microglial lines or in monocytes recruited to the CNS and was also observed in human microglia. Based on this signature, we found a crucial role for TGF-β in microglial biology that included: 1) the requirement of TGF-β for the in vitro development of microglia that express the microglial molecular signature characteristic of adult microglia; and 2) the absence of microglia in CNS TGF-β1 deficient mice. Our results identify a unique microglial signature that is dependent on TGF-β signaling which provides insights into microglial biology and the possibility of targeting microglia for the treatment of CNS disease.
Xenon gas treatment to restore microglial functions in neurodegenerative diseases
Abstract
Administrative Supplement Application PA-20-272 Xenon gas treatment to modulate microglia in neurodegenerative diseases (R41AG073059) ABSTRACT ABSTRACT FROM ORIGINAL APPLICATION Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder. Emerging evidence shows that homeostatic dysregulation of the brain immune system, especially that orchestrated by microglia, plays a significant role in the onset and progression of the disease. The microglial function is maintained in healthy brain and is pathogenically dysregulated in AD brain. The prominent genetic risk factors, APOE, is involved in microglial function. We have recently identified a unique molecular signature for homeostatic microglia and have developed robust tools to investigate microglial biology in health and disease. We also identified a role for the APOE-signaling in the regulation of a new microglial subset associated with neurodegeneration and in microglia surrounding neuritic Aβ-plaques in human AD brain, which we have termed MGnD. The major question relates to microglia-based approach to treat AD is how to modulate microglia phenotype and function. The goal of the original proposal was to investigate the Xenon (Xe) gas treatment to modulate microglia in AD mouse models and human iPSCs-derived microglia transplanted in humanized AD mice. Xe is currently used in human patients as an anesthetic and as a neuroprotectant in treatment of brain injuries. Xe penetrates blood brain barrier, which can make it effective therapeutic. Our original specific aims were as follow: Aim 1: Investigate whether Xe-gas treatment affects phenotype and function of neurodegenerative microglia in APP-PS1 mice. Aim 2: Validate whether Xe-gas treatment affects phenotype and function of neurodegenerative human microglia. SUPPLEMENTAL APPLICATION INFORMATION: To make competitive Phase II application and transition to clinical trial, we are planning to hold a pre-IND meeting with FDA. Developing such pre-IND meeting application was not a part of the original Phase I specific aims, but is closely connected. This new administrative supplement request will increase the likelihood to achieve additional critical R&D milestones in the technology development pathway to make us more competitive for Phase II application and ultimately for raising private-sector capital. The proposed scope of this additional supplement is within the overall scientific scope of the parent grant, which we will be able to complete by end of August 2022. The pre-IND submission package should include results of the originally proposed work and should address the following new specific aims: Aim 1: Determine PK/PD of Xenon inhalation treatment in an acute model of neurodegeneration and in APP/PS1 mice. The primary goal of our current efforts is to translate Xenon technology for testing in human AD patients. To prepare a competitive Phase II application for translation to humans, it is crucial to establish parameters of dosing based on the PK/PD. In this Aim, we will determine PK/PD of Xenon inhalation treatment in acute model of neurodegeneration and in AD mice. Aim 2: Identify blood biomarkers in myeloid cells from healthy and AD patients to monitor Xenon treatment. In this Aim, to monitor the efficacy of the Xe-treatment, we will determine the effect of Xenon treatment on blood immune cells. The results of these new proposed experiments will establish blood biomarkers for Xenon treatment and will be a crucial part for FDA submission and Phase I clinical trial. Feasibility and timeframe: Considering our capabilities and already completed work, we believe that these new important additional aims can be accomplished within the timeframe of the original grant and will take three months. Importantly, the MGnD acute neurodegenerative model, which has been developed in our lab, can be completed within 16hr. A new cohort of APP/PS1 mice has been generated, and the mice will be at 2 months of age (early onset of disease) and will be ready to be tested for PK/PD (Aim 1). All human samples from HC and AD patient are in hand (Aim 2).